Abstract
Introduction
Intravenous (IV) daratumumab has become a standard in the treatment of MM and AL amyloidosis largely due to its significant clinical benefit. Due to the high risk of infusion-related reactions (IRRs) prolonged infusion times are required at time of treatment initiation. These lengthy administrations can limit clinic capacity, require split dose infusions, increase chair time, and decrease patient satisfaction. Fixed-dose subcutaneous (SC) formulation of daratumumab (daratumumab and hyaluronidase-fihj) was approved in 2020 for the treatment of MM and subsequently, AL amyloidosis in 2021. While landmark trials have demonstrated its efficacy, there is lack of consensus around the standardized pre-medications and post-injection monitoring times for SC daratumumab. We present real world evidence from a large academic center with adoption and standardization of SC formulation of daratumumab into clinical practice.
Methods
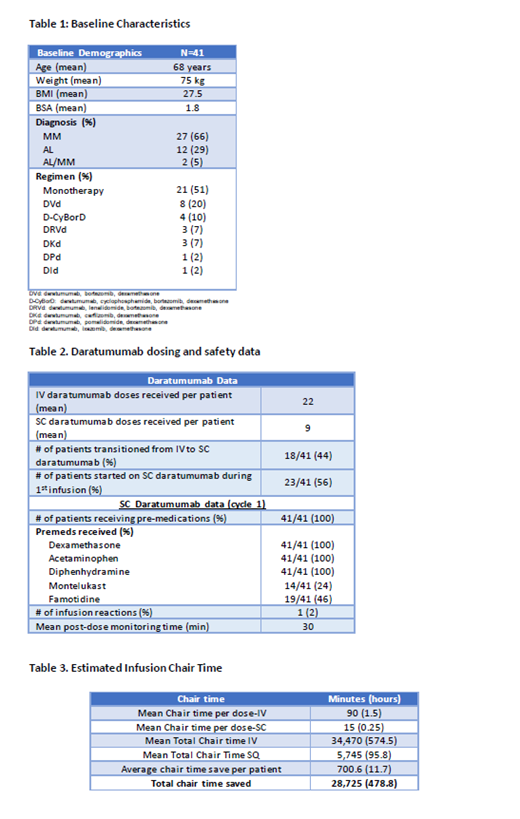
We evaluated all patients that received SC daratumumab for MM and/or AL amyloidosis at Boston Medical Center from June 1, 2020 to February 28, 2021. Baseline demographics were collected, including patient diagnosis, chemotherapy regimen, prior daratumumab administration details, and total doses of daratumumab received. Patients that were naïve to daratumumab, started their first dose as a SC injection. Patients were monitored for 30 minutes following the first SC dose and were pre-medicated with all oral agents: acetaminophen, dexamethasone, and diphenhydramine. Infusion chair time, improvement in clinic efficiency (using our standard infusion time of 90 minutes for IV daratumumab infusions after the 2nd dose), and safety were evaluated.
Results
A total of 41 patients were treated with SC daratumumab. Eighteen patients (44%) were switched from IV daratumumab to SC daratumumab. All other patients were naïve to SC daratumumab (n=23). All patients were monitored for 30 minutes after their first SC daratumumab dose only. In the absence of an ARR (administration-related reactions), patients were not monitored after subsequent injections. One patient had facial and neck swelling 2 days after administration of SC daratumumab (with no other symptoms) but resolved within 24 hours of an additional dose of dexamethasone and did not recur upon re-challenge of SC daratumumab. One patient experienced nausea following her first dose of SC daratumumab but it resolved without intervention. All patients received dexamethasone 20-40 mg (as anti-myeloma dose), acetaminophen 650 mg, and diphenhydramine 25-50 mg prior to the dose of SC daratumumab. Fourteen patients (24%) received montelukast 10 mg and 19 patients (46%) received famotidine 20-40 mg prior to the dose of SC daratumumab at provider discretion. Dexamethasone 4 mg daily for two days post-injection was not administered with SC formulation as was previously routine with the IV formulation. When analyzing the chair time saved by this standard monitoring protocol, a total of 478.8 hours of chair time were saved that were used for other patients in our practice (average of 11.7 hours saved per patient).
Conclusion
We report a successful conversion and adoption of SC daratumumab at our ambulatory hematology/oncology clinic. A standard 30-minute monitoring parameter can safely be implemented following the first SC daratumumab dose. Furthermore, less aggressive supportive medications can also be used. Integration of the SC formulation yielded significant chair time savings and may have the ability to create a more efficient clinic workflow.
Hughes: Rigel: Other: Advisory Board, Research Funding; Amgen: Speakers Bureau; Karyopharm: Other: Advisory Board, Speakers Bureau; Abbvie: Speakers Bureau. Sloan: Pharmacosmos: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria; Stemline: Honoraria. Sanchorawala: Abbvie: Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Prothena: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding; Caelum: Membership on an entity's Board of Directors or advisory committees, Research Funding; Regeneron: Membership on an entity's Board of Directors or advisory committees; Proclara: Membership on an entity's Board of Directors or advisory committees; Oncopeptide: Research Funding; Karyopharm: Research Funding; Sorrento: Research Funding; Pfizer: Honoraria.